Electron Configuration For Phosphorus 3-
2.six: Arrangements of Electrons
- Folio ID
- 16177
- Depict how electrons are grouped inside atoms.
Although we accept discussed the full general system of subatomic particles in atoms, nosotros have said little about how electrons occupy the infinite about the nucleus. Practice they move around the nucleus at random, or practice they be in some ordered arrangement?
The modern theory of electron behavior is called quantum mechanics. It makes the post-obit statements nigh electrons in atoms:
- Electrons in atoms can have merely certain specific energies. We say that the energies of the electrons are quantized.
- Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n ). Generally the higher the free energy of a shell, the farther it is (on boilerplate) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a college-energy vanquish volition spend more time farther from the nucleus than does an electron in a lower-free energy beat.
- Shells are further divided into subsets of electrons chosen subshells. The offset crush has but one subshell, the second shell has 2 subshells, the third beat out has three subshells, and so on. The subshells of each shell are labeled, in lodge, with the letters due south , p , d , and f . Thus, the first shell has only a unmarried s subshell (chosen 1 s ), the second crush has 2 s and 2 p subshells, the 3rd trounce has three southward , 3 p , and 3 d and so forth.
| Beat | Number of Subshells | Names of Subshells |
|---|---|---|
| one | 1 | 1s |
| ii | two | 2s and 2p |
| 3 | 3 | 3s, 3p and 3d |
| 4 | iv | 4s, 4p, 4d and 4f |
- Different subshells hold a different maximum number of electrons. Any s subshell can concord up to 2 electrons; p , 6; d , 10; and f , 14.
| Subshell | Maximum Number of Electrons |
|---|---|
| s | 2 |
| p | 6 |
| d | 10 |
| f | xiv |
Information technology is the arrangement of electrons into shells and subshells that most concerns us here, and then nosotros volition focus on that.
We use numbers to indicate which shell an electron is in. As shown in Table \(\PageIndex{1}\), the first crush, closest to the nucleus and with the everyman-free energy electrons, is shell i. This get-go shell has only i subshell, which is labeled one s and can concur a maximum of 2 electrons. We combine the shell and subshell labels when referring to the system of electrons well-nigh a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the southward subshell of the offset beat, nosotros use ane s 1 to describe the electronic construction of hydrogen. This construction is called an electron configuration. Electron configurations are autograph descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen cantlet is spoken out loud as "one-ess-ane."
Helium atoms accept two electrons. Both electrons fit into the ane south subshell because s subshells tin agree up to 2 electrons; therefore, the electron configuration for helium atoms is 1 south 2 (spoken as "one-ess-ii").
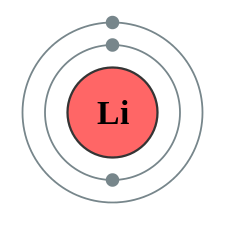
The one south subshell cannot concur 3 electrons (considering an s subshell tin hold a maximum of 2 electrons), so the electron configuration for a lithium atom cannot be 1 due south iii. Two of the lithium electrons tin can fit into the ane s subshell, but the third electron must go into the second shell. The 2d shell has two subshells, due south and p , which fill with electrons in that order. The 2 south subshell holds a maximum of 2 electrons, and the two p subshell holds a maximum of six electrons. Because lithium'southward last electron goes into the 2 s subshell, we write the electron configuration of a lithium atom as ane s 2ii s 1. The beat out diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has ii dots representing the two electrons in 1 s , while the outermost beat out (2 s ) has ane electron.
The side by side largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s twoii s 2. Now that the two s subshell is filled, electrons in larger atoms showtime filling the 2 p subshell. Thus, the electron configurations for the next six atoms are every bit follows:
- B: 1 s 22 s 22 p 1
- C: 1 southward two2 s iitwo p two
- Due north: 1 s 22 s 22 p 3
- O: 1 s 22 s 2two p 4
- F: 1 s 2two s 2ii p five
- Ne: 1 south 22 southward 22 p 6
With neon, the 2 p subshell is completely filled. Because the second shell has only 2 subshells, atoms with more electrons now must begin the third shell. The tertiary vanquish has three subshells, labeled s , p , and d . The d subshell can concur a maximum of 10 electrons. The first ii subshells of the 3rd vanquish are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1 s 2ii s 22 p 63 due south ii3 p 1. Notwithstanding, a curious thing happens afterwards the 3 p subshell is filled: the 4 s subshell begins to fill before the 3 d subshell does. In fact, the exact ordering of subshells becomes more than complicated at this signal (after argon, with its 18 electrons), and so we volition not consider the electron configurations of larger atoms. A fourth subshell, the f subshell, is needed to consummate the electron configurations for all elements. An f subshell can concur upwardly to 14 electrons.
Electron filling e'er starts with 1 due south , the subshell closest to the nucleus. Side by side is 2 s , two p , 3 s , 3 p , 4 south , iii d , 4 p , 5s, 4d, 5p, 6s, etc., shown in the electron crush filling gild diagram in Effigy \(\PageIndex{2}\). Follow each pointer in order from top to bottom. The subshells you reach along each arrow give the ordering of filling of subshells in larger atoms.
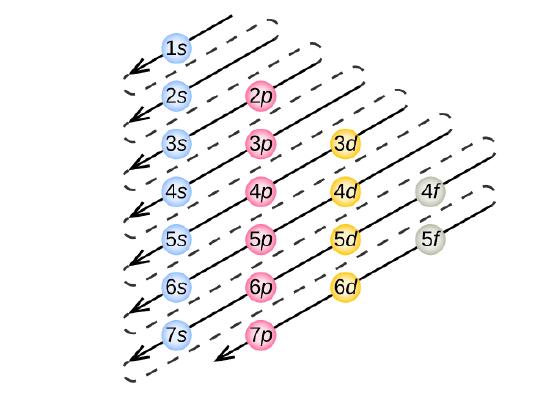
Using Figure \(\PageIndex{two}\) equally your guide, write the electron configuration of a neutral phosphorus cantlet. The atomic number of P is fifteen.
Solution
A neutral phosphorus atom has xv electrons. 2 electrons tin can go into the anesouth subshell, 2 tin can go into the 2s subshell, and half-dozen can get into the 2p subshell. That leaves v electrons. Of those 5 electrons, 2 can become into the 3s subshell, and the remaining iii electrons tin can go into the iiip subshell. Thus, the electron configuration of neutral phosphorus atoms is 1due south 2twos 2twop half dozenthreesouth iiiiip 3.
Using Effigy \(\PageIndex{ii}\) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of Cl is 17.
- Reply
-
A neutral chlorine atom has 17 electrons. Two electrons tin can go into the 1south subshell, 2 tin can go into the 2southward subshell, and 6 can go into the iip subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the iiis subshell, and the remaining 5 electrons tin can become into the iiip subshell. Thus, the electron configuration of neutral chlorine atoms is isouth ii2s 22p 6threes ii3p5 .
Since the arrangement of the periodic tabular array is based on the electron configurations, Figure \(\PageIndex{3}\) provides an alternative method for determining the electron configuration. The filling order simply begins at the top left, with hydrogen (Z=1) and includes each subshell as you proceed in increasing diminutive number (Z) club.
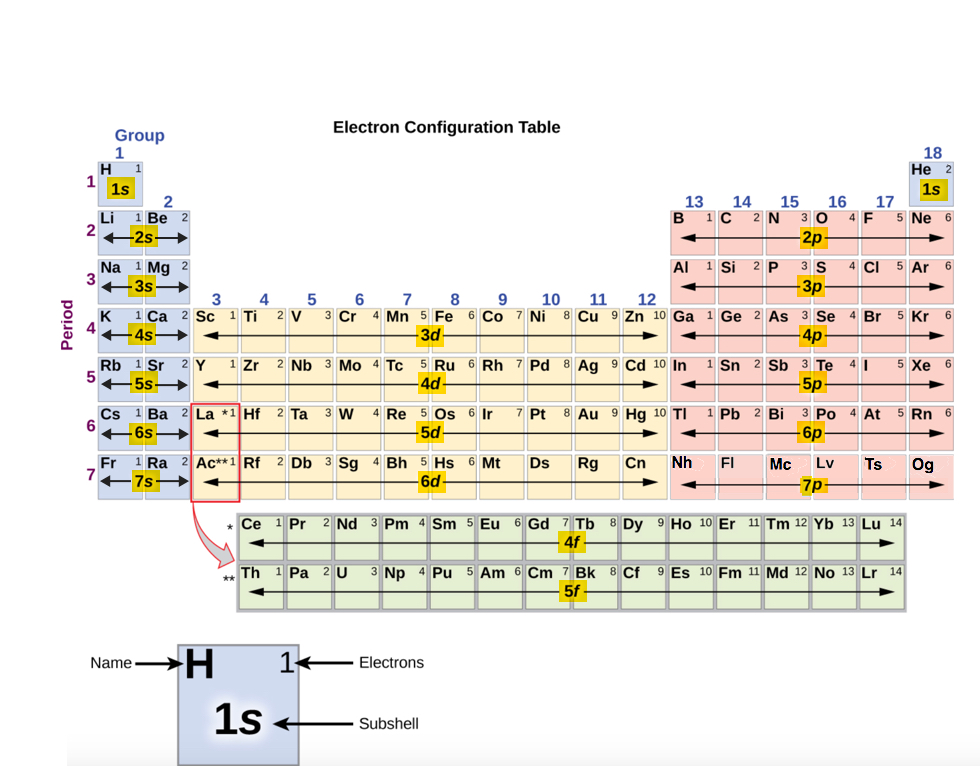
For instance, the start row (Menstruum 1) contains H and He only, because only two electrons are required to fill the 1s subshell. The second row s-block, contains simply two elements, Li and Be, to fill the 2s subshell. This is followed past the second row p-cake, containing half dozen elements (B through Ne) since six electrons are required to fill up the 2p subshell. The third row is like to the second row elements. 2 electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell. After filling the 3p block up to Ar, nosotros see the next subshell volition exist 4s (K, Ca), followed by the 3d subshell, which are filled by 10 electrons (Sc through Zn). The 4p subshell is filled next by six electrons (Ga through Kr). Every bit y'all can see, the periodic table shown in Figure \(\PageIndex{iii}\) provides a simple manner to retrieve the club of filling the subshells in determining the electron configuration. The society of filling subshells is the same: 1s, 2 southward , ii p , iii s , 3 p , four s , three d , four p , 5s, 4d, 5p, 6s, etc.
Using Figure \(\PageIndex{three}\) equally your guide, write the electron configuration of neutral aluminum atom. The atomic number of Al is 13.
Solution
Aluminum has thirteen electrons.
Commencement at Period 1 of the periodic table, Figure \(\PageIndex{iii}\). Place two electrons in the 1s subshell (1sii ).
Keep to Menstruation 2 (left to right direction). Place the next 2 electrons in the 2s subshell (2s2 ) and the next six electrons in the 2p subshell (2p6 ).
Proceed to Menstruation 3 (left to right direction) . Place the next two electrons in the 3s subshell (3stwo ) and the last one electron in the 3p subshell (3pi ).
The electron configuration of Aluminum is 1s22sii2p63stwo3p1
Practise \(\PageIndex{two}\)
Using Figure \(\PageIndex{three}\) every bit your guide, write the electron configuration of the atom that has 20 electrons
- Answer
-
First at Period 1 of Figure \(\PageIndex{3}\). Identify ii electrons in the 1s subshell (1s2 ).
Proceed to Period 2 (left to right direction). Identify the next ii electrons in the 2s subshell (2s2 ) and the next half dozen electrons in the 2p subshell (2phalf dozen ).
Proceed to Catamenia three (left to correct direction) . Identify the side by side two electrons in the 3s subshell (3stwo ) and the next vi electron in the 3p subshell (3p6 ).
Proceed to Menses iv. Place the remaining two electrons in the 4s subshell (4s2).
The electron configuration is 1s22stwo2psix3s23phalf dozen4s2
Valence Electrons
In the report of chemical reactivity, we volition find that the electrons in the outermost principal energy level are very of import and then they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an cantlet.
In the second catamenia elements, the two electrons in the \(1s\) sublevel are called inner-crush electrons and are not involved directly in the chemical element'south reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You lot must recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels and and then the answer is three. In fact, the number of valence electrons goes upwardly by one for each step across a period until the last element is reached. Neon, with its configuration ending in \(2s^ii 2p^vi\), has eight valence electrons.
The brine metal sodium (diminutive number eleven) has i more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3 due south orbital, giving a 1 s 22 south 2ii p 63 s 1 configuration. The electrons occupying the outermost beat orbital(s) (highest value of n ) are called valence electrons, and those occupying the inner shell orbitals are called core electrons ( Figure \PageIndex4). Since the core electron shells stand for to noble gas electron configurations, nosotros can abbreviate electron configurations by writing the noble gas that matches the cadre electron configuration, along with the valence electrons in a condensed format. For our sodium example, the symbol [Ne] represents core electrons, (i s 2two s 22 p half dozen) and our abbreviated or condensed configuration is [Ne]iii s 1.

Similarly, the abbreviated configuration of lithium can be represented every bit [He]2s 1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the brine metal family unit, accept but ane electron in a valence due south subshell exterior a filled set up of inner shells.
\[\ce{Li:[He]}\,2s^1\\ \ce{Na:[Ne]}\,3s^1 \nonumber \]
A chemical reaction results from electron removal, electron addition, or electron sharing of the valence electrons of the different atoms. The path a specific element will take depends on where the electrons are in the atom and how many there are. Thus, information technology is convenient to separate electrons into two groups. Valence shell electrons (or, more only, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s 2iidue south 22p 2—that it has 4 valence electrons (2south 22p 2) and 2 core electrons (1southward 2). You will see in the side by side chapters that the chemical backdrop of elements are determined by the number of valence electrons.
Examine the electron configuration of neutral phosphorus atoms in Example \(\PageIndex{ane}\), 1southward 22s 22p 6iiisouth 2iiip three and write the abbreviated notation.
Solution
Phosphorus has electron configuration, 1s 22s two2p sixthrees 2iiip 3.
The highest-numbered shell is the third trounce (threesouthward iithreep 3): two electrons in the 3southward subshell and 3 electrons in the 3p subshell. That gives a total of 5 valence electrons.
The ten inner trounce (core) electrons, is 22s 22p 6 can be replaced by [Ne] (see Effigy \(\PageIndex{three}\)). Abbreviated notation is : [Ne]threes 23p 3
Examine the electron configuration of neutral calcium atom (Exercise \(\PageIndex{2}\)), anes 22s iitwop six3s 23pvi 4sii , and write the abbreviated annotation.
- Answer
-
The highest-numbered beat out is the quaternary beat 4s2, which has 2 electrons in the 4s subshell. Hence, Calcium has 2 valence electrons.
The 18 inner-crush (core) electrons, is 2twodue south 22p viiiis 2iiip6 , can exist replaced by [Ar], run into Figure \(\PageIndex{three}\). The abbreviated note is: [Ar]4s2
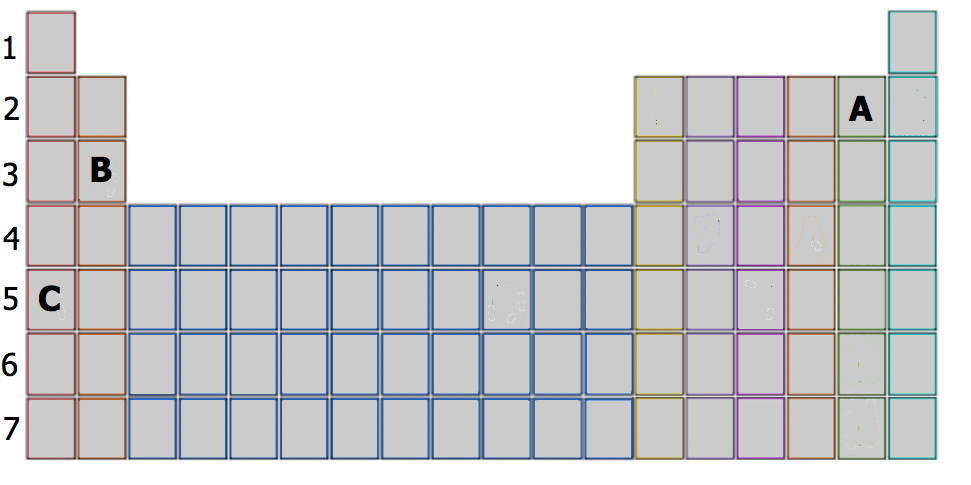
Based on their respective locations in the periodic table (employ Figure \(\PageIndex{3}\)), make up one's mind the number of valence electrons and the valence shell configuration of elements A, B and C.
Solution
Element A is located in Period two, the 5th position in 2p-block. Before the electrons are placed in 2p subshell, the 2s subshell must be filled beginning. This ways that A has two valence electrons in 2s (2s2 ) and 5 valence electrons in 2p (2p5 ). Answer: 2s22p5. It has two + 5 = 7 valence electrons.
Element B is located in Catamenia three, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2 ). Answer: 3stwo .
Element C is located in Period 5, the 1st position in 5s-block). This means that at that place is only one valence electron in 5s (5si ). Answer: 5si .
Do \(\PageIndex{four}\)
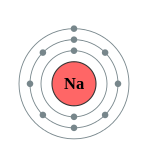
Using the location of Na is the periodic table (Figure \(\PageIndex{3}\)), depict the shell diagram of sodium atom.
- Answer
-
Sodium (Na) is the first element in the 3rd row (Period 3) in the periodic table. This means that the first trounce and second shells of Na cantlet are filled to the maximum number of electrons.
The get-go crush (1s) is filled with 2 electrons. The second shell (2s and 2p) has a total of 8 electrons . And, the third (terminal) shell has 1 electron .
The shell diagram of the Na atom is shown beneath. The shell nearest the nucleus (first shell) has 2 electrons (2 dots), the second trounce has 8 electrons and the last (outermost) beat has 1 electron. (2.8.1)
Concept Review Exercises
- How are electrons organized in atoms?
- What data does an electron configuration convey?
- What is the difference between core electrons and valence electrons?
Answers
- Electrons are organized into shells and subshells around nuclei.
- The electron configuration states the arrangement of electrons in shells and subshells.
- Valence electrons are in the highest-numbered beat; all other electrons are core electrons.
Key Takeaway
- Electrons are organized into shells and subshells most the nucleus of an atom.
- The valence electrons determine the reactivity of an atom.
Exercises
Electron Configuration For Phosphorus 3-,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Basics_of_General_Organic_and_Biological_Chemistry_(Ball_et_al.)/02%3A_Elements_Atoms_and_the_Periodic_Table/2.06%3A_Arrangements_of_Electrons
Posted by: pierrewasell.blogspot.com


0 Response to "Electron Configuration For Phosphorus 3-"
Post a Comment